Update on St. Luke's Inductions
Subject: 42 weeks!
As of September 1st St Lukes will be offering inductions up to 42 weeks!
I will be happy to go over details and questions with any of you but wanted you to know right away.
Some details:
Cnms can take care of these patients
We recommend continuous monitoring
We recommend ANT at 41 and at 41.3-41.4.
Vbac is next. Stay tuned.
Love
Becca Amirault and the whole gang at St Lukes.
Cesarean Birth Pie
From Science and Sensibilty: https://www.scienceandsensibility.org/blog/series-brilliant-activities-for-birth-educators-cesarean-pie?source=1&utm_source=feedburner&utm_medium=email&utm_campaign=Feed%3A+science-sensibility+%28Science+%26+Sensibility%29
Overall, 43.2 percent of the women who were given the typical three hours to push ended up having a C-section. But just 19.5 percent of the women who were given four hours had one.
Full article: New Evidence That We Just Need To Give Women More Time To Labor
When moms in a recent study had one more hour to push, the C-section rate was cut in half.
The time that a woman spends in the second stage of labor — when she pushes, then finally delivers her baby — is among the most intense stretches of her life, and is monitored extremely closely. The American College of Obstetricians and Gynecologists says that first-time moms generally have three hours to push their baby out if they’ve had an epidural, two if they haven’t, and beyond that, they’re thought to be experiencing a prolonged second stage of labor. Healthcare providers may move for a C-section, or an assisted delivery with a vacuum or forceps.
But a new study found that when women were given just one more hour to push, C-section rates went down by roughly half. And while the investigation — published in the March issue of the American Journal of Obstetrics and Gynecology — is small and unlikely to fundamentally change medical norms any time soon, researchers say it offers a much-needed critique of potentially outdated standards.
“[The time recommendation] came from expert opinion from the 1800s,” said Dr. Alexis Gimovsky, a fellow in maternal fetal medicine at Thomas Jefferson University in Pennsylvania, and an author on the study. “Since then, there’s really only been retrospective data used to validate that guideline.” In the 1950s, researchers looked over earlier data and found that women who delivered their babies within two hours had lower rates of infection and serious postpartum bleeding, for example. In 1955, another team concluded that most women without anesthesia give birth within two hours.
The new study included 78 first-time moms delivering at Thomas Jefferson University Hospital, who were randomly assigned to have either the usual time limit of three hours, or allowed an extra hour to push. (The researchers looked only at women who had epidurals, not because that’s what they set out to do, but because they simply did not have any patients who did not get pain meds and who qualified for the study.)
“There can be benefits to allowing women to labor longer.”
Overall, 43.2 percent of the women who were given the typical three hours to push ended up having a C-section.
But just 19.5 percent of the women who were given four hours had one.
“The study really showed what we’ve seen in practice for years, which is that there can be benefits to allowing women to labor longer,” said Gimovsky. “We were excited to see that it dramatically reduced the risk of C-section in this specific group of women.”
The researchers also found no evidence that giving women more time put them, or their babies, at greater risk — though Gimovsky cautioned that the study was too small to truly capture potential harms. Prior research has found risks to mother and baby when labor goes on too long, she said, so this is an area that requires further investigation.
Of course, for many women and babies, C-sections are absolutely vital. The World Health Organization has long advocated that countries’ C-section rates not exceed 10-15 percent of births, but a recent study found that maternal and infant mortality rates continue to decline in countries as C-section rates reach up to 19 percent. The surgery saves lives.
The aim of the new study is not to challenge that fact, but rather help ensure that the current guidelines most doctors rely on are based in clear, strong evidence. Currently, more than 30 percent of births in the U.S. each year are C-sections, and Gimovsky said that around 10-15 percent of those take place in cases where the second stage of labor has stalled.
And prior investigations have likewise examined how simply giving women more time in labor may help eliminate some surgical births. One found, for example, that women today tend to take longer in labor than 50 years ago. Why isn’t yet understood. But as NPR reported, an implication of the finding is that “today’s obstetricians may be rushing to do Cesarean sections too soon because they’re using an out-of-date yardstick for how long a ‘normal’ labor should take.”
“It’s OK to ask to see if you’re a candidate for waiting longer.”
For now, pregnant women should feel empowered to talk to their care providers about their options, Gimovsky said — understanding, of course, that there are times when intervention is necessary.
“A woman can have a conversation with her doctor during labor if the primary reason for a C-section is the length of time ... it’s OK to ask to see if you’re a candidate for waiting longer,” she said. “It’s something so simple that can make a major difference in your life.”
Limiting food intake during labor (even with an epidural) is not evidenced based care
10.24.15 - Link to full article here
SAN DIEGO – Most healthy women can skip the fasting and, in fact, would benefit from eating a light meal during labor, suggests research being presented at the ANESTHESIOLOGY® 2015 annual meeting. Improvements in anesthesia care have made pain control during labor safer, reducing risks related to eating, researchers note.
Women traditionally have been told to avoid eating or drinking during labor due to concerns they may aspirate, or inhale liquid or food into their lungs, which can cause pneumonia. But advances in anesthesia care means most healthy women are highly unlikely to have this problem today and when researchers reviewed the literature of hundreds of studies on the topic, they determined that withholding food and liquids may be unnecessary for many women in labor.
“Our findings suggest a change in practice makes sense,” said Christopher Harty, BN, co-author of the study and a medical student at Memorial University, St. John’s, Newfoundland, Canada. “Physician anesthesiologists and obstetricians should work together to assess each patient individually. Those they determine are at low risk for aspiration can likely eat a light meal during labor. This gives expectant mothers more choices in their birthing experience and prevents them from being calorie deficient, helping to provide energy during labor.”
Researchers said aspiration today is almost nonexistent, especially in healthy patients. In the United States, there was only one case of aspiration associated with labor and delivery between 2005 and 2013, involving a complicated case of a woman who was obese and had pre-eclampsia (a precursor to eclampsia, or high blood pressure that can lead to seizures), according to the American Society of Anesthesiology’s Closed Claims Project database. Researchers also noted that no cases of death due to aspiration were reported in the United Kingdom between 2000 and 2005, compared to 1.5 cases per 1,000 during the 1940s. They say this is likely due to advances in anesthesia care, including increased use of epidurals and spinal blocks in place of providing anesthesia through a mask over the nose and mouth. Before these improvements, women were more likely to need a tube placed in the windpipe for breathing, which potentially increased the risk of aspiration.
Researchers analyzed 385 studies published in 1990 or later that focused on women who gave birth in a hospital. The research suggests that the energy and caloric demands of laboring women are similar to those of marathon runners, Harty said. Without adequate nutrition, women’s bodies will begin to use fat as an energy source, increasing acidity of the blood in the mother and infant, potentially reducing uterine contractions and leading to longer labor and lower health scores in newborns. Additionally, the studies suggest that fasting can cause emotional stress, potentially moving blood away from the uterus and placenta, lengthening labor and contributing to distress of the fetus.
“However, certain factors increase a laboring patient’s risk of aspiration which outweigh the risks of withholding nutrition,” said Erin Sprout, BN, co-author of the study and a medical student at Memorial University. These factors include eclampsia, pre-eclampsia, obesity and the use of opioids to manage labor pain, which delays stomach emptying, she said.
Healthy women who are not at risk for aspiration should ask their medical care providers (including their physician anesthesiologist and obstetrician) if eating a light meal during labor is safe for them. A light meal could include fruit, light soups, toast, light sandwiches (no large slices of meat), juice and water. Most women lose their appetites during very active labor, but can continue to drink fluids such as water and clear juices, researchers said.
2014 SF and KRWC Cesarean Rates
The Leapfrog Group, a non-profit organization promoting hospital safety standards, has published 2014 cesarean rates by hospital in low-risk 1st-time mothers (> 37 wk, one, head-down baby)
CPMC - 21.7%
Kaiser SF - 20.3%
St. Luke's - 21.1%
UCSF - 21.5%
Kaiser Redwood City - 13.8%
SFGH - did not report
What does the Human Placenta Project mean for obstetrics care?
By: MARY ELLEN SCHNEIDER, Ob.Gyn. News Digital Network
Earlier this year, officials at the National Institutes of Health launched the Human Placenta Project with the aim of better understanding how this organ functions and what role it plays in adverse pregnancy outcomes from preeclampsia to preterm birth.
One of the main goals of the project is to develop new technologies for the real-time assessment of placental development, allowing for the study of placental function in normal versus abnormal pregnancies. NIH officials also seek to develop noninvasive markers to predict adverse pregnancy outcomes and better understand how the placenta affects long-term health. In late September, the agency took a step toward those goals by awarding $46 million to researchers around the country.
What does this mean for obstetrics care? In an interview, Dr. Catherine Y. Spong, a maternal-fetal medicine specialist and deputy director of the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., explained how this initiative is different from studying the placenta after birth, as well as when this research could yield clinically meaningful results.
Question: Through the project, you’re setting out to study the human placenta in real time. What will that tell us that looking at the placenta at birth alone does not?
Dr. Spong: Just as the fetus develops and changes across pregnancy, the placenta does as well. Early on, the placenta invades into the uterine decidua, invades the spiral arteries, and replaces the endothelium to make the vessels large bore, for easy flow of blood to the fetus. If this does not happen, preeclampsia may develop and the fetus may not grow appropriately.
Using current methods of evaluating the placenta after delivery does not help us determine how the placenta is working or functioning early or in mid-gestation. It is akin to doing an autopsy at the end of life and trying to figure out how the person developed and acted during their youth and midlife.
Our hope is that by monitoring the placenta in action, we will gain a much richer understanding of normal placental function – and of placental problems that can lead to common pregnancy complications.
Question: What could this research potentially tell us about hard-to-prevent conditions such as preterm birth and other adverse pregnancy outcomes?
Dr. Spong: Preterm birth and adverse pregnancy outcomes have as one component abnormal placental function. If the placenta cannot support the pregnancy, the baby may not grow well and may develop growth restriction. The placenta, if not formed appropriately, also may result in preeclampsia (toxemia), stillbirth, or preterm birth. If we could better understand how the placenta develops and functions in healthy pregnancies, we may be able to spot potential problems earlier on and test interventions to see if they improve outcome.
Question: How will environmental impacts on pregnancy be monitored through the placenta?
Dr. Spong: It is clear that environmental influences affect outcomes. One of the initiatives we are supporting will help to evaluate how these influences affect outcome. Investigators will study factors such as smoking or obesity, thus including women who do and who do not have these influences, and determine how they affect placental development.
Question: The NIH is investing more than $40 million in this project. How will the money be spent?
Dr. Spong: Our funds will support development of novel approaches for placental assessment, research on environmental influences that affect placental structure and function, and interdisciplinary collaborations, including investigators new to placental research who bring important expertise in bioimaging and biotechnology.
We have been impressed by the proposals we have received, both in their quality and breadth. Investigators are focusing on a range of imaging technologies, including MRIs and ultrasound, as well as techniques to identify biomarkers of placental function in maternal blood samples. Investigators also are interested in many different environmental factors, from nutrition to air pollution.
This initial investment in the Human Placenta Project should lead to new approaches to safe, noninvasive, real-time monitoring of placental development and function across pregnancy, and yield important insights into environmental influences on placental health.
Question: It takes a while for research findings to translate into clinical practice. How long before ob.gyns. can see practice-changing recommendations as a result of this project?
Dr. Spong: This is an important question, as not only do we need the evidence to determine what is important for normal placental development and function, but we also need to learn what additional steps to take to optimize pregnancy outcome. The timing for this – both identifying the development, structure, and function of the placenta across human gestation and determining how best to intervene and optimize outcome – may take some time. However, we believe that this work will be time well spent. It is clear that pregnancy affects the lifelong health of both the mom and the fetus. If we could optimize pregnancy outcome, we could improve the health of the nation.
Find more information on the NIH’s Human Placenta Project here.
Examination of placentas from prolonged pregnancies shows no evidence of any increased incidence of gross placental abnormalities
Aging of the placenta
It is widely believed that during the relatively short duration of a normal pregnancy the placenta progressively ages and is, at term, on the verge of a decline into morphological and physiological senescence.1-3 This belief is based on the apparent convergence of clinical, structural, and functional data, all of which have been taken, rather uncritically, as supporting this concept of the placenta as an aging organ with, all too often, no distinction being made between time related changes and true aging changes. I will review some of these concepts and consider whether the placenta truly undergoes an aging process. For the purposes of this review an aging change is considered to be one which is intrinsic, detrimental, and progressive and which results in an irreversible loss of functional capacity, an impaired ability to maintain homeostasis, and decreased ability to repair damage.
Morphological changes
The placenta is unusual in so far as its basic histological structure undergoes a considerable change throughout its lifespan. For some time it has been customary to describe the appearances of the placental villi in terms of their changing appearance as pregnancy progresses, comparing, for instance, typical first trimester villi with those in third trimester placentas. It has often been implied that this changing appearance is an aging process, but it is now recognised that this temporal variability in villous appearances reflects the continual development and branching of the villous tree (fig 1) In recent years the relation between the growth of the villous tree and the villous histological appearances has been formally codified5-8with identification of five types of villi (fig 2).
Diagrammatic representation of a peripheral villous tree, showing a large central stem villus: the lateral branches from this are the mature intermediate villi from which the terminal villi protrude.
Representation of the peripheral branches of a mature villous tree together with typical cross sections of the five villous types. The figures are reproduced from Haines & Taylor. Textbook of Obstetrical and Gynaecological Pathology. 4th Edn. 1995, by kind permission of Churchill Livingstone and Professor P Kaufmann.
1 Mesenchymal villi
These represent a transient stage in placental development and they can differentiate into either mature or immature intermediate villi. They comprise the first generation of newly formed villi and are derived from trophoblastic sprouts by mesenchymal invasion and vascularisation. They are found mainly in the early stages of pregnancy but a few may still be found at term They have complete trophoblastic mantles with many cytotrophoblastic cells and regularly dispersed nuclei in the syncytiotrophoblast: their loose, immature-type stroma is abundant and contains a few Hobauer cells, together with poorly developed fetal capillaries.
2 Immature intermediate villi
These are peripheral extensions of the stem villi and are the predominant form seen in immature placentas. These villi have a well preserved trophoblastic mantle in which cytotrophoblastic cells are numerous; the syncytial nuclei are evenly dispersed and there are no syncytial knots or vasculo-syncytial membranes. They have an abundant loose stroma that contains many Hofbauer cells: capillaries, arterioles, and venules are present.
3 Stem villi
These comprise the primary stems which connect the villous tree to the chorionic plate, up to four generations of short thick branches and further generations of dichotomous branches. Their principal role is to serve as a scaffolding for the peripheral villous tree, and up to one third of the total volume of the villous tissue of the mature placenta is made up of this villous type, the proportion of such villi being highest in the central subchorial portion of the villous tree. Histologically, the stem villi have a compact stroma and contain either arteries and veins or arterioles and venules; superficially located capillaries may also be present.
4 Mature intermediate villi
These are the peripheral ramifications of the villous stems from which most terminal villi directly arise. They are large (60–150 μm in diameter) and contain capillaries admixed with small arterioles and venules, the vessels being set in a very loose stroma which occupies more than half of the villous volume. The syncytiotrophoblast has a uniform structure, no knots or vasculo-syncytial membranes being present. Up to a quarter of the villi in a mature placenta are of this type.
5 Terminal villi
These are the final ramifications of the villous tree and are grape-like outgrowths from mature intermediate villi. They contain capillaries, many of which are sinusoidally dilated to occupy most of the cross sectional diameter of the villus. The syncytiotrophoblast is thin and the syncytial nuclei are irregularly dispersed. Syncytial knots may be present and vasculo-syncytial membranes are commonly seen. These terminal villi begin to appear at about the 27th week of gestation and account for 30–40 per cent of the villous volume, 50 per cent of the villous surface area, and 60 per cent of villi seen in cross section at term.
The pattern of development of the villous tree is therefore as follows: During the early weeks of pregnancy all the villi are of the mesenchymal type. Between the 7th and 8th weeks mesenchymal villi begin to transform into immature intermediate villi and these subsequently transform into stem villi. Development of additional immature intermediate villi from mesenchymal villi gradually ceases at the end of the second trimester, but these immature intermediate villi continue to mature into stem villi and only a few persist to term as growth zones in the centres of the lobules. At the beginning of the third trimester mesenchymal villi stop transforming into immature intermediate villi and start transforming into mature intermediate villi. The latter serve as a framework for the terminal villi which begin to appear shortly afterwards and predominate at term.
This progressive elaboration of the villous tree results in a predominance of terminal villi in the mature placenta. Such villi have been conventionally classed as “third trimester villi” and a comparison of their structure with the predominant type of villi in the first trimester— immature intermediate villi—has led many to suggest that as pregnancy progresses the villous trophoblast becomes irregularly thinned and the cytotrophoblast regresses, changes interpreted as being of an aging nature. The villous cytotrophoblast, which is a stem cell for the trophoblast, does not in reality regress, because the absolute number of these cells in the placenta is not decreased at term and in fact continues to increase throughout pregnancy. The apparent sparsity of these cells is due to their wider dispersion within a greatly increased total placental mass.9 10The focal thinning of the villous syncytiotrophoblast apparent in many terminal villi has often been cited as evidence of syncytial senescence. These thinned areas are, in reality, the “vasculo-syncytial membranes”11 which, although formed in part by mechanical stretching of the trophoblast by ballooning capillary loops,12 never the less differ enzymatically and ultrastructurally from the non-membranous areas of the syncytium and are areas of the syncytiotrophoblast specifically differentiated for the facilitation of gas transfer.13These membranes are therefore a manifestation of topographic functional differentiation within the trophoblast.
The interlinked, but separate, processes of maturation of the villous tree and functional differentiation of the trophoblast result in a predominant villous form that is optimally adapted for materno-fetal transfer diffusion mechanisms: the morphological changes substantially increase trophoblastic surface area14 and a significantly reduce the harmonic mean of the diffusion distance between maternal and fetal blood,15 with a resulting increase in the conductance of oxygen diffusion.16
It is not mere pedantry to distinguish between maturation, which results in increased functional efficiency, and aging, which results in decreased functional efficiency. In this respect it is worth noting that a proportion of placentas from women with severe pre-eclampsia look unusually mature for the length of the period of gestation: this is usually classed as “premature aging” but it would be more accurate to regard the changes as being due to accelerated maturation, this being a compensatory mechanism to increase the transfer capacity of the placenta in the face of an adverse maternal environment.
It has to be admitted that the control mechanisms of placental maturation are unknown. There are many agents thought to be of importance in the control of placental growth, including cytokines, growth factors, oncogenes, prostaglandins and leucotrienes,17-20 but it far from clear as to whether control of growth can be equated with control of maturation. However, villous development, certainly in the later stages of pregnancy, does seem to be driven principally by proliferation of endothelial cells and capillary growth.21 Vascular endothelial growth factors are present in placental tissue22 and the suggestion that hypoxia may stimulate angiogenesis,23 and thus have a significant role in placental development, would corroborate the accelerated placental maturation seen in some cases of maternal pre-eclampsia.
Placental growth
It has long been maintained that placental growth and DNA synthesis cease at about the 36th week of gestation and that any subsequent increase in placental size is due to an increase in cell size rather than to an increase in the number of cells.24Simple histological examination of the term placenta will, however, serve to refute this view, because immature intermediate villi are often present in the centres of lobules and these clearly represent a persistent growth zone. Furthermore, total placental DNA content continues to increase in an almost linear manner until and beyond the 42nd week of gestation.25 This finding agrees with autoradiographic and flow cytometric studies that have shown continuing DNA synthesis in the term organ,26-28 and with morphometric investigations that have shown persistent villous growth, continuing expansion of the villous surface area, and progressive branching of the villous tree up to and beyond term.14 29
Placental growth certainly slows, but clearly does not cease, during the last few weeks of gestation, although this decline in growth rate is neither invariable nor irreversible, because the placenta can continue to increase in size if faced with an unfavourable maternal environment, such as pregnancy at high altitude, or severe maternal anaemia, while the potential for a recrudescence of growth is shown by the proliferative response to ischaemic syncytial damage. Those who contend that a decreased placental growth rate during late pregnancy is evidence of senescence often seem be comparing the placenta with an organ such as the gut, in which continuing viability depends on a constantly replicating stem cell layer producing short-lived postmitotic cells. A more apt comparison would be with an organ such as the liver, which is formed principally of long-lived postmitotic cells and which, once an optimal size has been attained to meet the metabolic demands placed on it, shows little evidence of cell proliferation while retaining a latent capacity for growth activity. There seems no good reason why the placenta, once it has reached a size sufficient to adequately meet its transfer function, should continue to grow, and the term placenta, with its considerable functional reserve capacity, has more than met this aim.
Functional activity
There have been few vertical studies of placental function throughout pregnancy, but there is no evidence that any of the major indices of placental function decline—namely, proliferative, transfer, and secretory capacities.30 As already remarked, the diffusion conductance of the organ is increased, largely as a result of morphological changes, but there is considerable evidence that specific placental carrier mediated transfer systems are also augmented.20 The placental production of hormones continues unabated until term: the synthesis of human chorionic gonadotrophin declines towards the end of the first trimester but this is clearly due to a gene switch which results in progressively increasing secretion of human placental lactogen.
The placenta also retains its full proliferative capacity until term as shown by its ability to repair and replace, as a result of proliferation in the villous cytotrophoblastic cells, of a villous syncytiotrophoblast that has been ischaemically damaged in women with severe pre-eclampsia.13
Clinical factors
The single most important factor leading to a belief in placental senescence has been the apparently increased fetal morbidity and mortality associated with prolonged pregnancy, this traditionally being attributed to “placental insufficiency” consequent on senescence of the organ.1 31 In the past it was thought that about 11% of pregnancies extended to or beyond the 42nd week of gestation32 33 : the introduction of a routine ultrasound examination in early pregnancy reduced the incidence of prolonged pregnancies to about 6%34 and it has even been claimed that with very accurate dating studies the incidence of truly prolonged gestations does not exceed 1%.35 This casts some doubt on the validity of a great deal of the historical information about the risks and ill effects of prolonged pregnancy, but it is never the less widely accepted that perinatal mortality increases after the 42nd week of gestation.36
Any increase in perinatal mortality after the 42nd week of gestation is due, in part, to the high incidence of fetal macrosomia: 10% of infants from prolonged pregnancies weigh over 4000 g and 1% over 4500 g and these fetuses are at particular risk of complications such as shoulder dystocia. The presence of this large number of macrosomic fetuses is a clear indication that, in this subset at least, the placenta continues to function well beyond the 40th week of gestation and remains capable of sustaining untrammelled fetal growth.
The classic clinical syndrome of the “postmature” infant1 31 is not commonly seen today but seems to be clearly related to the development of oligohydramnios. There is no doubt that amniotic fluid volume tends to decrease in a proportion of prolonged pregnancies39 and that oligohydramnios is associated with a high incidence of fetal heart rate decelerations.36 This has been attributed by some to cord compression,40 41 but one study, while confirming that cord compression is common in prolonged pregnancies, was unable to correlate such compression with fetal acidosis.42 It is often assumed, and indeed commonly stated, that the decline in amniotic fluid volume in these cases is an indication of “placental insufficiency” but, in reality, there is no evidence that in late pregnancy the placenta plays any part in the production of amniotic fluid or in the control of amniotic fluid volume.43
The two most potent causes of increased morbidity in prolonged pregnancy are therefore clearly unrelated to any change in placental functional capacity. Examination of placentas from prolonged pregnancies shows no evidence of any increased incidence of gross placental abnormalities, such as infarcts, calcification, or massive perivillous fibrin deposition. The most characteristic histological abnormality, found in a proportion of cases but certainly not in all, is decreased fetal perfusion of the placental villi.13The fetal villous vessels are normal in placentas from prolonged pregnancies44 and Doppler flow velocimetry studies have, in general but not unanimously, indicated that there is no increased fetal vascular resistance in such placentas.45-47 The decreased fetal perfusion is therefore probably a consequence of oligohydramnios, because umbilical vein flow studies have shown that fetal blood flow to the placenta is often reduced in cases of oligohydramnios.48
It has to be admitted that the pathophysiology of prolonged pregnancy has not been fully elucidated. It seems, however, quite clear that any ill effects which may befall the fetus in prolonged gestations can not be attributed to placental insufficiency or senescence.
Conclusions
A review of the available evidence indicates that the placenta does not undergo a true aging change during pregnancy. There is, in fact, no logical reason for believing that the placenta, which is a fetal organ, should age while the other fetal organs do not: the situation in which an individual organ ages within an organism that is not aged is one which does not occur in any biological system. The persisting belief in placental aging has been based on a confusion between morphological maturation and differentiation and aging, a failure to appreciate the functional resources of the organ, and an uncritical acceptance of the overly facile concept of “placental insufficiency” as a cause of increased perinatal mortality.
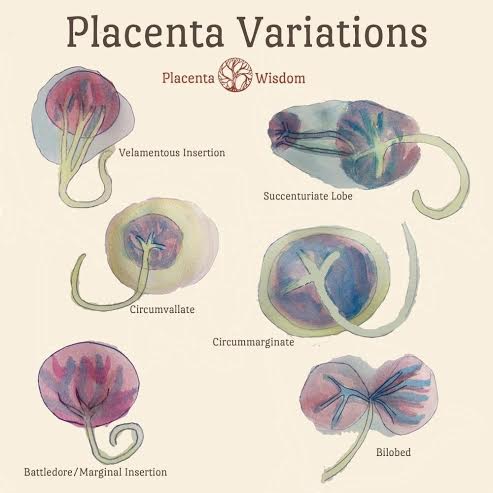
Placenta Research and Studies
Vascular Biology of the Placenta
Placentophagia in Humans and Non-human Mammals
Yale University School of Medicine Placenta Research
Fatigue as a Predictor of Postpartum Depression
Hormonal Changes in the Postpartum and Implications for Postpartum Depression
Iron Content of Intact Placentas and Cords
Maternal Iron Deficiency Anemia Affects Postpartum Emotions and Cognition
Placentophagia: A Biobehavioral Enigma
Enhancement of Opioid-Mediated Analgesia: A Solution to the Enigma of Placentophagia
Have we forgotten the significance of postpartum iron deficiency?
Role of Placenta in Nutrient Transfer
Wound Healing Activity of Human Placental Extracts in Rats
GMO Foods and Damage to Human Babies, Placentas, & Umbilical Cords
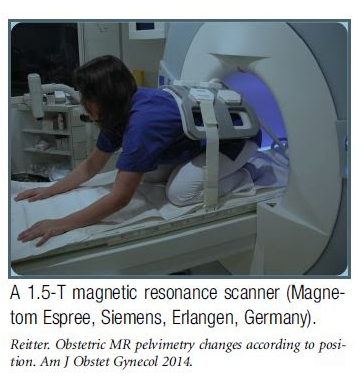
Posture Affects Pelvic Dimensions
If you've ever wondered whether positioning affects pelvic dimensions or whether this is an "old midwives" tale, wonder no more. A 2014 study found that it did. Investigators used obstetric magnetic-resonance imaging pelvimetry to measure pelvic dimensions in pregnant women and nonpregnant women in a kneeling squat (the machine would not allow measurements in a more upright position) vs. lying on their backs. The kneeling squat expanded the pelvis by a significant amount. What is more, the difference was more pronounced in pregnant women. Interestingly, the investigators were trying to determine optimal positioning for vaginal breech delivery. They note that current guidelines for vaginal breech assume that the woman is on her back and suggest that more upright postures might facilitate spontaneous birth of the aftercoming head, which could reduce maternal and newborn morbidity. - Henci Goer
Bacteria and Heavy Metals in the Placenta
Recent news stories, blog posts, and online articles have claimed that “science fails to see benefits of eating placenta.”1 Many of these articles have been fueled by papers published by researchers at Northwestern University. Courtney Durfee of the Association for the Placenta Arts Preparation (APPA) gave a thoughtful review to the two papers, viewable here.
Some of these articles include forceful concerns of heavy metals and harmful bacteria. A recent NY Times article states that “….the placenta is not sterile, and several studies found that the organ was contaminated with bacteria as well as selenium, cadmium, mercury and lead.”1 The article links two NIH studies as references, we will address both along with taking a deeper look at these worrisome statements.
We should begin with talking about how the placenta works and some of its roles to the mother and fetus. The placenta is often referred to as a filter; this isn’t an ideal term for the placenta considering its function in the body. As consumers we use filters in daily life to remove unwanted particles and toxins then throwing them into the trash once they have reached capacity. The placenta does not function as a filter in this sense, a more suitable way of viewing it would be as a gatekeeper between the mother and fetus. The placenta’s job is to keep the maternal and fetal blood separate, at the same time allowing nutrients to pass to the fetus, gas exchange to occur, and allowing waste from the fetus to pass through to the mother. The placenta does prevent some toxins from passing through to the fetus but they are not stored in the placenta. Toxins in the body and waste from the fetus are processed by the mother’s liver and kidneys for elimination.
Now that we have a better understanding of how the placenta works lets take a look at “the heavy metals found in the placenta.” The NIH study linked in the NY Times article reviewed past studies of mercury, cadmium, and lead levels found in the placenta worldwide.2 For a better understanding of what the data means we will focus on the most recent numbers listed for the United States. Starting with mercury, the review did not find a notable amount of mercury in the placentas of mothers giving birth in the US. Next, cadmium levels in the US showed an average of 4.4 ng/g with the highest levels reported from urban areas and levels two to three times higher amongst women who smoked during the pregnancy. The average placenta weighs 550 g, which would result in an average of 2.42 µg of cadmium in the entire placenta. The FDA does not regulate the levels of cadmium in food products. According to the European Food Safety Authority the average adult can consume 170 µg of cadmium per week safely, which is approximately 24 µg a day.3 So even if the mother were to consume the entire placenta in one day, the amount of cadmium would be significantly lower than the amount deemed safe by the EFSA. Lastly, the most recent US study in the review shows lead levels in the placenta averaging 5.1 ng/g. For a bit of perspective about what this number means, the FDA recommends that the level of lead should not exceed 100 ng/g in candy.4 Note: the NY Times article states that selenium is also found in the placenta, the linked research article did not take a look at or review any studies that referenced selenium. Selenium is not a metal; it’s a mineral and one that is shown to have positive effects on the body and developing fetus. A study conducted in 2012 showed a correlation between delivering SGA infants (small-for-gestational-age) and lower levels of selenium in the placenta.5
These three metals cross the placental barrier to the developing fetus and also once the baby is born through breast milk. The consensus amongst medical professionals is that the benefits of an infant consuming breast milk greatly outweighs any risks that these heavy metals may or may not have on the developing infant.6 These heavy metals are a part of our environment, foods, and have become something that our bodies contain. One could also suggest that the benefits of placenta consumption outweigh any minimal risks to the mother.
Now, lets address “the placenta is not sterile and is contaminated with bacteria.” Yes, the placenta is not sterile, no part of our body is. There are very few things that exist in the world that are sterile and they are often created by man to be this way for a short period of time. Our world is full of bacteria, many of which are beneficial. In a study, also funded by the National Institute of Health, Dr. Kjersti Aagaard of Baylor College of Medicine states “the infant is exposed to several environmental sources of bacteria in the early neonatal interval through the maternal vaginal canal and feces, swallowing and breathing, skin to skin contact, maternal breastmilk, etc.” She reports that exposure to the bacteria during and immediately following the birth process is beneficial to building a microbiome.7 Some believe this process is so vital that researchers are now studying the use of vaginal swabs to ‘seed’ babies born by C-section and telling new moms to skip that first bath on the day of birth. So yes, your placenta and baby have been “contaminated” by the bacteria in and around your vagina during birth.
There are lots of good this bacteria exposure can do, can some bacteria that the placenta is exposed to be harmful? While there have been no research findings that suggest there are harmful bacteria routinely contained within the placenta prior to birth, it is possible that the placenta can come in contact with bacteria that may pose a risk for illness if consumed. If opting to have your placenta encapsulated it important to find a Certified Placenta Arts Specialist trained in Food Safety. Just like with the foods we eat, proper handling, storage, and processing are key steps to the mother’s safety. There are times when its not advisable for a mother to consume her placenta, such as when an intrauterine infection is present, and these concerns should be discussed with their doctor or midwife at the birth.
We agree whole-heartedly that more research about human placenta consumption and encapsulation is needed. We are excited to know that there will be more information available in the coming years. In the meantime, we hope to see more news articles that are not fear-based but focus on education. Information that supports mothers wishing to consume their placenta, to keep any risks associated minimal and that reinforces the proper steps for safety. We encourage women to do their research and ask qualified professionals questions to ensure that they are making informed decisions about their care and the handling of their placenta following the birth.
Post written and researched by Nikole Keller who owns LifeTree Placenta Services in New Jersey. She serves as a board member for the Association of Placenta Preparation Arts (APPA) focusing on furthering safe placenta processing methods.
Sources:
$10 Weekly Lactation Clinic & Breastfeeding Support Group
Facilitated by Lactation Consultants
Sign up or email: cwlactation@gmail.com for more info
Fascinating Placenta Statistics
Raw Foods Method (dehydrated at 115F): For every gram of raw placenta processed via this method you will get 0.376 size 0 capsules. That means for an average size placenta (400g) you will get 150 capsules.
USDA Jerky Method (dehydrated at 160F): For every gram of raw placenta processed via this method you will get 0.342 size 0 capsules. That means for an average size placenta (400g) you will get 137 capsules.
TCM (steamed, dehydrated at 125F): For every gram of raw placenta processed via this method you will get 0.288 size 0 capsules. That means for an average size placenta (400g) you will get 115 capsules.
Much gratitude for Emily Fontes for tracking and sharing these statistics.
Autism risk spotted at birth in abnormal placentas
Researchers at the Yale School of Medicine have figured out how to measure an infant’s risk of developing autism by looking for abnormalities in his/her placenta at birth, allowing for earlier diagnosis and treatment for the developmental disorder. The findings are reported in the April 25 online issue of Biological Psychiatry.
One out of 50 children are diagnosed with an autism spectrum disorder in the United States each year, according to the Centers for Disease Control and Prevention (CDC), but the diagnosis is usually made when these children are 3 to 4 years of age or older. By then the best opportunities for intervention have been lost because the brain is most responsive to treatment in the first year of life.
Senior author Dr. Harvey Kliman, research scientist in the Department of Obstetrics, Gynecology & Reproductive Sciences at the Yale School of Medicine, and research collaborators at the MIND Institute at the University of California, Davis, have found that abnormal placental folds and abnormal cell growths called trophoblast inclusions are key markers to identify newborns who are at risk for autism.
Kliman and his team examined 117 placentas from infants of at-risk families, those with one or more previous children with autism. These families were participating in a study called Markers of Autism Risk in Babies – Learning Early Signs. Kliman compared these at-risk placentas to 100 control placentas collected by the UC Davis researchers from the same geographic area.
The at-risk placentas had as many as 15 trophoblast inclusions, while none of the control placentas had more than two trophoblast inclusions. Kliman said a placenta with four or more trophoblast inclusions conservatively predicts an infant with a 96.7% probability of being at risk for autism.
Currently, the best early marker of autism risk is family history. Couples with a child with autism are nine times more likely to have another child with autism. Kliman said that when these at-risk families have subsequent children they could employ early intervention strategies to improve outcomes. “Regrettably couples without known genetic susceptibility must rely on identification of early signs or indicators that may not overtly manifest until the child’s second or third year of life,” said Kliman.
“I hope that diagnosing the risk of developing autism by examining the placenta at birth will become routine, and that the children who are shown to have increased numbers of trophoblast inclusions will have early interventions and an improved quality of life as a result of this test,” Kliman added.
This work was supported by the National Institutes of Health, the U.S. Environmental Protection Agency through the Science to Achieve Results (STAR) program, the MIND Institute at the University of California, Davis, and the Yale University Reproductive and Placental Research Unit.
http://news.yale.edu/2013/04/25/autism-risk-spotted-birth-abnormal-placentas